








Purity: 99.5%min. HPLC
Spec:5mg 10mg 15mg 20mg 30mg 60mg(Customizable)
Appearance: White Crystalline Lyophilized Powder
Storage: Refrigeration keeps dry and away from light.
Categories: Weight Loss
Prodyct name: Control Weight
Specification: 5mg, 10mg, 15mg, 20mg, 30mg, 60mg(customizable)
Purity: 99.50%
Appearance: White Crystalline Lyophilized Powder
Typical use: Weight loss
Shelf Life: 24 Months
Storage: Refrigeration keeps dry and away from light.
This product is a weight management auxiliary preparation developed based on modern metabolic science, specially designed for the need of healthy fat loss. It supports the entire process of weight management through a multi-target mechanism of action.
TR, standard specifications: 5mg, 10mg, 15mg, 20mg, 30mg, 50mg, 60mg.
Supports customization including 100mg, color, bottle body, etc.
Core Functional Features
Metabolic regulatory system
Support the balance of sugar and lipid metabolism
Promote the efficiency of energy conversion
Maintain the basal metabolic rate
Appetite Management System
Regulate the signal transmission of satiety
Balance appetite-related hormones
Reduce the urge to eat that is not physiological
Optimization of digestion and absorption
Improve the efficiency of nutrient utilization
Regulate the intestinal microecological environment
Promote the excretion of metabolic wastes







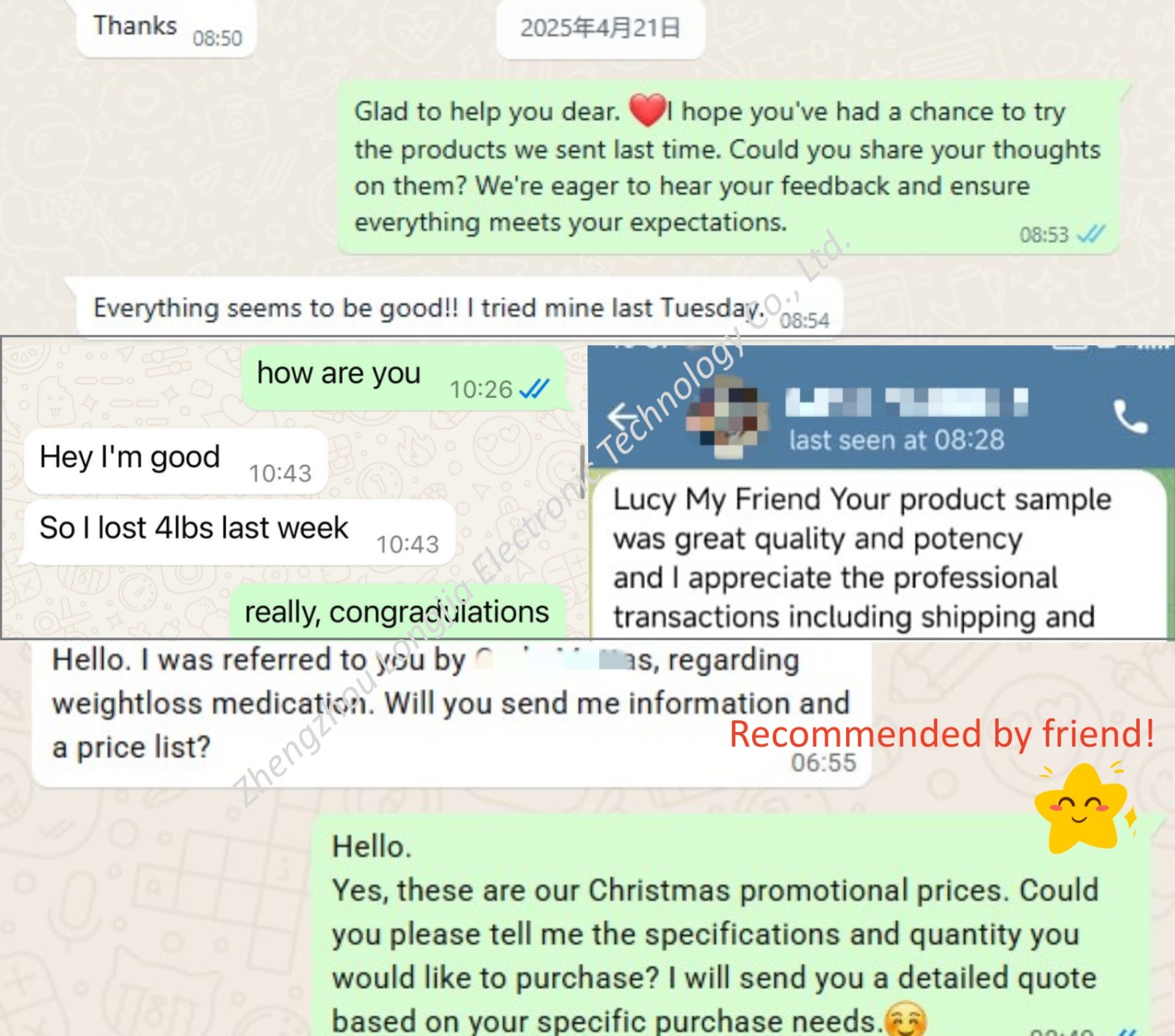
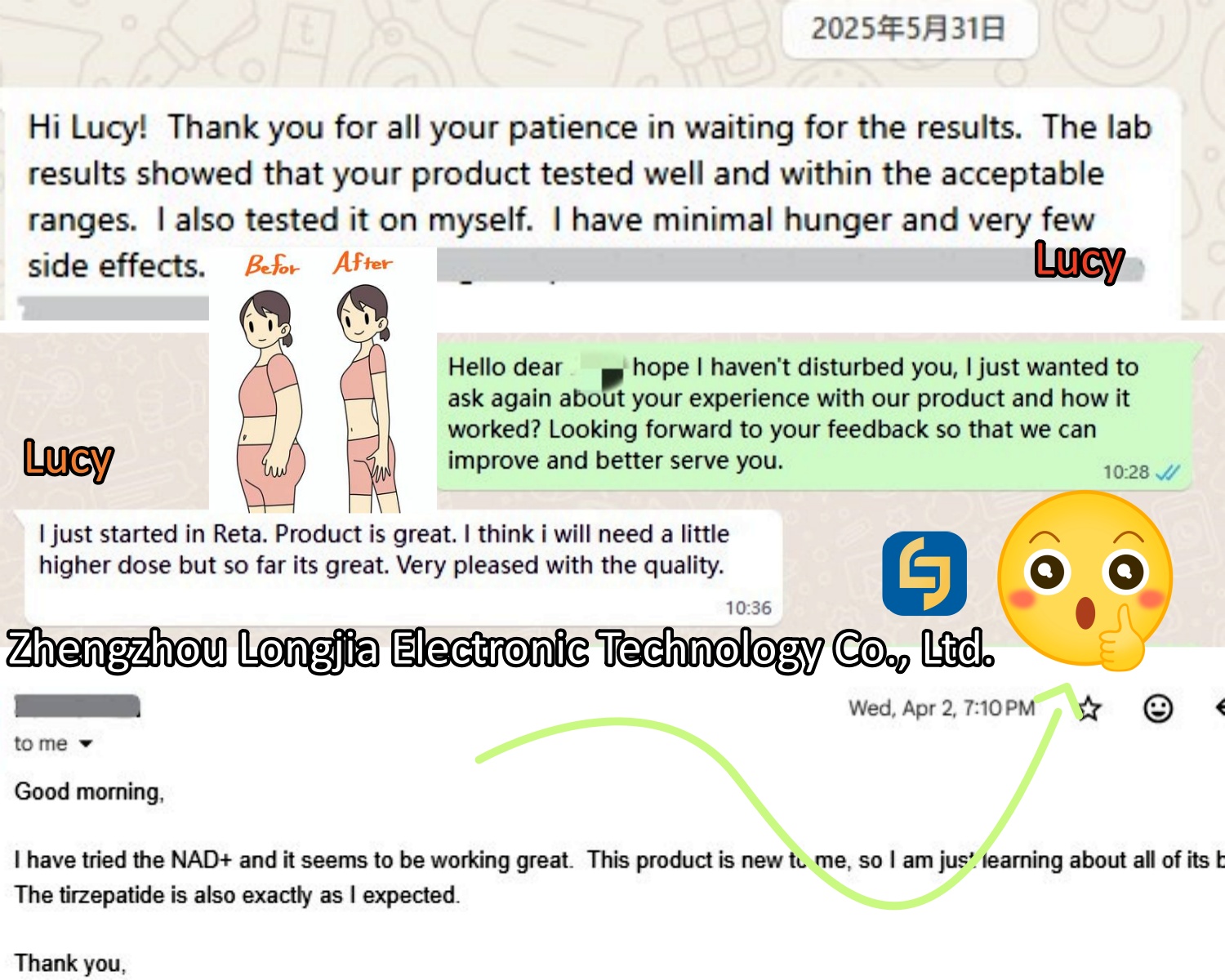
Zhengzhou Longjia Electronic Technology Co., Ltd. is a professional pharmaceutical science and technology company, dedicated to the research, development, production and sales of peptides, pharmaceutical raw materials and fine chemical intermediates. In terms of product quality control, our company adheres to a strict quality management system. From raw material procurement, production process to product inspection, each link is strictly controlled to ensure the high quality and stability of our products. As a company facing the global market, our products are exported to many countries and regions, and have won wide praise and trust from customers. We always adhere to the principle of ‘quality first, customer first’, and take customers as the centre to provide personalized product customization and solutions to meet customers’ different needs.
Our range of slimming peptides is the result of years of extensive research and collaboration with leading scientists in the field of biochemistry. By utilizing the latest advances in peptide therapy and cutting-edge manufacturing technologies, we have been able to create products that deliver real, measurable results.
In addition to their effectiveness, Longjia′s peptides are formulated with the highest standards of safety and purity. We employ rigorous testing and quality control processes to ensure that every product we offer is of the highest quality. All of our peptides are manufactured in state-of-the-art facilities that comply with international standards, and our production processes adhere to strict quality assurance protocols.
If you′re looking for a reliable and effective solution to support your weight management journey, Zhengzhou Longjia Electronic Technology Co., Ltd. is your trusted partner. Contact us today to learn more about our products and how they can help you achieve your goals.





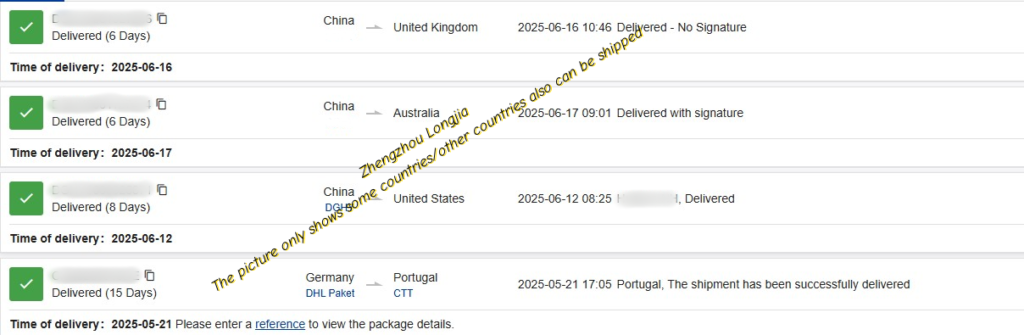
High quality, competitive price, adequate stock and fast delivery competitive price.
1. Enterprise standard and 99% purity.
2. Exclusive one-to-one customer service, any inquiries will be replied within 12 hours.
3. Safe and fast shipping, about 7-15 days to your address.
4. Tracking number provided to you after ordering, keep tracking goods and update information to you until the goods arrive safely at your address.
5. OEM service, redesign label, packing box as customer’s requirement. Add customer’s logo.
6. Excellent after-sale service.
Q1: How is the product quality?
A: Pharmaceutical raw material products strictly select raw materials. We guarantee that the concentration of each box of product is higher than 99%. Support third-party testing, if the test does not meet the standard, we will replace the product for free or support the refund.
Q2: Do you provide samples?
A: Yes, we provide samples for free.
Q3: Is there any discount
A: Yes, for larger quantity, we always support with better price.
Q4: What payment terms do you accept?
A: We accept Bank Transfer, Wise, PayPal, Western Union, Bitcoin and USDT etc.
Q5: When can I receive the goods after payment?
A: Depending on your location
For small order, please expect 5-7 days by DHL,UPS,TNT, FEDEX, EMS.
or mass order, please allow 5-15 days by Air, 20-35 days by Sea.
Q6: How do you treat quality complaint?
A: First of all, our quality control will ensure the product quality in advance. If there is a real quality problem caused by us, we will send you free goods for replacement or refund.
Company slogan: Innovation, Cooperation, Win Win
Company Introduction: Zhengzhou Longjia Electronic Technology Co., Ltd. is a professional pharmaceutical technology company dedicated to the research and development, production, and sales of peptides, pharmaceutical raw materials, pharmaceutical intermediates, and fine chemical intermediates.
Company team: Zhengzhou Longjia Electronic Technology Co., Ltd. has a strong technical background, and the team members are all doctoral, master's, and undergraduate students. They are skilled in process route design, synthesis, process optimization, and new drug research for drugs and chemical intermediates. At the same time, the company is equipped with various advanced HPLC-MS, GC-MS instruments and organic synthesis detection equipment, which can meet the detection needs from milligrams to kilograms.
Company slogan: Innovation, Cooperation, Win Win
Company Introduction: Zhengzhou Longjia Electronic Technology Co., Ltd. is a professional pharmaceutical technology company dedicated to the research and development, production, and sales of peptides, pharmaceutical raw materials, pharmaceutical intermediates, and fine chemical intermediates.
Company team: Zhengzhou Longjia Electronic Technology Co., Ltd. has a strong technical background, and the team members are all doctoral, master's, and undergraduate students. They are skilled in process route design, synthesis, process optimization, and new drug research for drugs and chemical intermediates. At the same time, the company is equipped with various advanced HPLC-MS, GC-MS instruments and organic synthesis detection equipment, which can meet the detection needs from milligrams to kilograms.




In the future, the company will continue to be committed to "doing fine, specialized and stronger", and strive to build an advanced professional manufacturing base in the industry.




Copyright © 2024 Zhengzhou Longjia Electronic Technology Co., Ltd. All Rights Reserved. Privacy Policy